
A thimbleful of liquid heavy-hydrogen fuel could produce as much energy as 20 tons of coal. You need that input energy (the match), but what you get as a result is far more powerful. Fusion is like lighting a match to a bucket of gasoline.
In fact, both the extra neutron and the new helium nucleus (called an alpha particle) carry off excess energy which can be used (to heat water, for example). In the fusion reaction a deuterium and tritium atom combine together, or fuse, to form an atom of helium and an energetic neutron.It only takes a small amount of these isotopes to produce a lot of energy! The deuterium-tritium fusion reaction results in an energy gain of about 450:1!! No other energy source we can tap releases so much energy for the amount that is input. Tritium can be bred from lithium, which is abundant in the earth's crust. Deuterium can be easily extracted from seawater, where 1 in 6500 hydrogen atoms is deuterium. The first generation fusion reactors will use deuterium and tritium for fuel because they will fuse at a lower temperature. You can see the fusion process happening with these two nuclei, in the diagram at the top of the page. One is called deuterium, the other tritium. similar forms of hydrogen, but with extra neutrons in their nuclei. The simple hydrogen atom, which has one proton in its nucleus, has two isotopes. The nuclei used by the sun, and in experiments on earth, that undergo fusion, are two isotopes of hydrogen called deuterium and tritium. The energy is 'extra' because the mass of the newly formed nucleus is less than the sum of the masses of the original two nuclei the extra mass is converted to energy according to Einstein's equation E=mc2 This energy can be used to do useful work! When the nuclei fuse, they form a new element, and release excess energy in the form of a fast-moving neutron. high enough to overcome the strong repulsive forces of the respective protons in the nuclei. We will try to show how fusion works, and describe current efforts to tame this limitless energy source.įusion is what happens when two atomic nuclei are forced together by high pressure. It is what provides the sun and the stars with the energy to shineĬontinuously for billions of years. Fusion has been used here on earth to produce nuclear bombs, but has not yetīeen controlled so that we can obtain useful energy. Nuclear fusion is the energy source of the future. Therefore, the total mass does decrease a tiny bit during the reaction. Because energy and mass are one and the same, the energy released is also mass released. Thus, when the uranium atom is split, some of the energy that held it together is released as radiation in the form of heat. Extra mass is a result of the binding energy that holds the protons and neutrons of the nucleus together. The mass of an atom is more than the sum of the individual masses of its protons and neutrons, which is what those numbers represent. Thus, it seems that no mass is converted into energy. The mass seems to be the same on both sides of the reaction: You might have been wondering, "Where does the energy come from?".
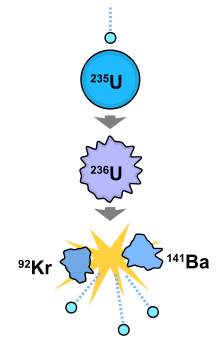
We gave the following equation as an example:Ģ35U + 1 neutron -> 2 neutrons + 92Kr + 142Ba + ENERGY In the section above we described what happens when an 235U atom fissions. In this animation, one can see how the fissioning of each 235U atom (red) releases more neutrons (green) that go on to fission more 235U atoms, thus producing a chain reaction. This is roughly what happens in an atomic bomb. The chain reaction will continue until all the 235U fuel is spent. Those 4 neutrons can strike 4 more 235U atoms, releasing even more neutrons. Each of those atoms releases 1 more neutron bringing the total neutrons to 4. If more 235U is present, those 2 neutrons can cause 2 more atoms to split. When the atom is split, 1 additional neutron is released. In each of the above reactions, 1 neutron splits the atom. * 235U + 1 neutron -> 2 neutrons + 92Sr + 140Xe + ENERGY * 235U + 1 neutron -> 2 neutrons + 92Kr + 142Ba + ENERGY The following two equations are examples of the different products that can be produced when 235U fissions: However, the products' masses always add up to 236. The fissioning of 236U can produce over twenty different products.
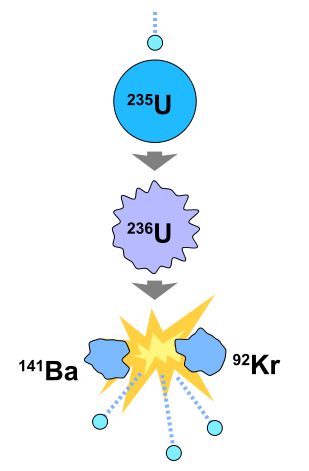
236U is unstable and this causes the atom to fission. When a stray neutron strikes a 235U nucleus, it is at first absorbed into it. Uranium is the principle element used in nuclear reactors and in certain types of atomic bombs. Are your customers raving about you on social media? Share their great stories to help turn potential customers into loyal ones.


 0 kommentar(er)
0 kommentar(er)
